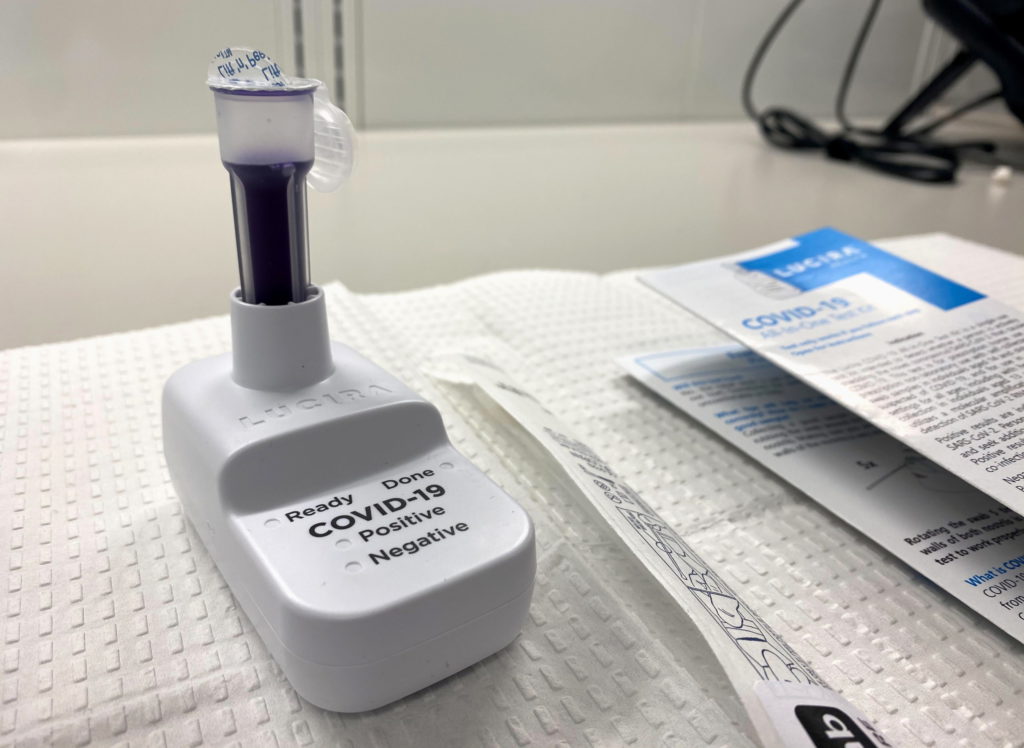
The U.S. Food and Drug Administration said on Tuesday it had approved the first COVID-19 self-testing kit for home use that provides results within 30 minutes.
The single-use test, made by Lucira Health, has been given emergency use authorization for home use with self-collected nasal swab samples in individuals aged 14 and older who are suspected of COVID-19 by their health care provider, the FDA said.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn said.
The kit can also be used at hospitals and point-of-care settings but samples should be collected by a healthcare provider if the individuals who are tested are younger than 14 years, the health regulator said.
Although a recent string of positive news from Moderna Inc and Pfizer Inc on their potential vaccines has raised hopes in combating the disease, testing still is a key factor in controlling the spread of the virus.
“We look forward to proactively working with test developers to support the availability of more at-home test options,” said Jeff Shuren, the director of the FDA’s Center for Devices and Radiological Health.
The United States crossed 11 million total infections on Sunday