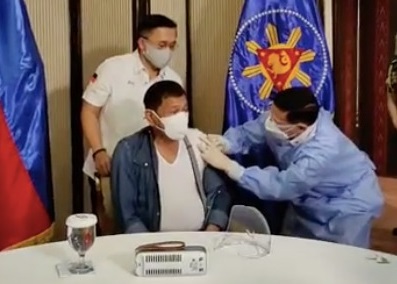
MANILA, Philippines — In a rare response to public criticism, President Rodrigo Duterte admitted it was wrong to get himself inoculated with the Sinopharm vaccine and ordered the return of the remainder of the 1,000 doses donated by China.
The President, however, will still get his second shot of the vaccine as one dose will be kept for him, presidential spokesperson Harry Roque said on Thursday, May 7, 2021.
But even if Duterte decides not to take the next jab of Sinopharm’s BBIBP-CorV, it would be safe for him to get his second dose from the vaccine made by the Chinese pharmaceutical Sinovac Biotech called CoronaVac, according to a member of the government’s vaccine expert panel.
Health Secretary Francisco Duque III administered the vaccine to the President on Monday, May 3.
Duterte later apologized for receiving the Sinopharm shot after he was criticized on social media for getting inoculated with a vaccine that has yet to secure an emergency use authorization (EUA) from the Food and Drug Administration (FDA).
An EUA, which Sinopharm has not applied for, indicates that the regulator had studied a particular drug and determined it to be efficacious and safe for use in an emergency such as the current pandemic.
“Well, we are sorry that we committed the things that you are criticizing us for,” Duterte said during his nationally televised address on Wednesday night. “We accept responsibility. I myself had been vaccinated. Well, it’s the decision of my doctor. Anyway, it’s my life.”
The President, however, said that his inoculation was legal because the Presidential Security Group (PSG) in February received a compassionate special permit (CSP) from the FDA to use it.
“When the government allowed it to be used for compassionate use, that itself was an authority for people to be injected. But only a few have been given this,” he said.
“So here’s a deal,” he said, “I told the ambassador that they criticized this since Sinopharm did not undergo examination. I said, just remove these. You withdraw all Sinopharm vaccines, 1,000 of them. Do not send Sinopharm here so that there would be no trouble. I said just give us Sinovac which is used on everybody.”
He was referring to China’s ambassador to the Philippines. The Chinese Embassy did not immediately respond to the Inquirer’s request for comment.
Jose Santiago Sta. Romana, the Philippine ambassador to China, refused to comment on the decision of the President to return the Sinopharm vaccine, referring questions on it to Roque.
Roque explained on Tuesday that the President decided to have himself inoculated with the Sinopharm vaccine under the CSP because the EUA for it “has been grossly delayed.”
Secretary Carlito Galvez Jr., chief implementer of the National Task Force Against COVID-19 and the official in charge of the national vaccination program, on Tuesday said the 1,000 doses of the Sinopharm vaccine arrived last month along with the 500,000 shots of CoronaVac purchased by the government.
No WHO endorsement
The Sinopharm vaccine has become controversial in the Philippines after members of the PSG, particularly the President’s personal security detail, inoculated themselves with it in September-October last year, even ahead of its approval by China’s own state regulator.
The World Health Organization (WHO) has yet to endorse the safety and efficacy of BBIBP-CorV, which is produced by the state-owned company China National Pharmaceutical Group, or Sinopharm, and Beijing Bio-Institute of Biological Products Co. Ltd.
The Sinopharm vaccine, with a 79.34 percent efficacy rate, was authorized by the China National Medical Products Administration on Dec. 31, 2020.
It was among the first vaccines deployed in China’s emergency use program and had been used to inoculate millions of people.
Both Sinopharm and Sinovac use inactivated viruses in producing their vaccines, which allow the body to create antigens. These antigens then signal the body’s immune system to attack the SARS-CoV-2 virus, which causes the severe respiratory disease COVID-19.
“If for example, Sinopharm is no longer an option, there is another similar vaccine to Sinopharm that has an emergency use authorization. That is Sinovac,” Rontgene Solante, a member of the vaccine expert panel, said in an online briefing.
He explained that since the two vaccines used the same development platform, Sinovac’s CoronaVac could be used for Duterte’s second shot, Solante said.
FDA chief Dr. Eric Domingo on Tuesday said the PSG was not cooperating with its investigation of the unauthorized use of the Sinopharm vaccine last year.
He told the Inquirer that in addition to the 10,000 doses for which the PSG received a permit for compassionate use, the presidential guards also wanted an additional 1,000 doses for their civilian staff.
“I think they used the vaccines for the civilians on the President,” he said.
Cabral: FDA pressured
Former Health Secretary Esperanza Cabral said Duterte’s inoculation was “legalized” by the CSP.
But the issuance of the CSP was made “after the fact” since some of the President’s personal bodyguards were “illegally” vaccinated with the Sinopharm vaccine as early as September, Cabral pointed out.
“Well, that is something the FDA is going to have to regret because this is not something that regulatory agencies should be doing – acceding to pressure and legitimizing and regularizing illegal activities,” she said, adding that the regulator would have to repeat the same thing later.
Cabral said the FDA might have been under severe political pressure.
“If (Domingo) feels that he cannot do what his superior authority wants him to do … because he feels that is wrong, that is illegal, the only alternative is for him to resign,” she said.