EXPLAINER: The whos and hows in the process of Covid vaccine approval
MANILA, Philippines — As our country tries to join other nations in combatting Covid-19 through inoculation programs, news about separate acquisition projects and agreements of the national and local government with vaccine manufacturers might confuse some Filipinos.
Others might wonder who are the people or agencies in charge of the whole vaccine trials and procurements. While some might ask how and what are the important processes the doses have to go through before the vaccines reach the people?
Who are the officials and agencies involved in the decision-making?
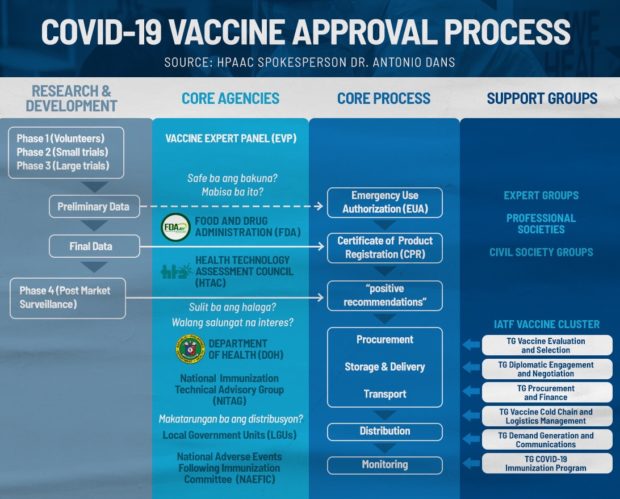
In the Philippines, there are at least eight different agencies among the core agencies that perform separate functions during the process of approving a Covid-19 vaccine.
DOST-VEP, SJERB, FDA
The first one on the list is the Vaccine Expert Panels (VEP) under the Department of Science and Technology (DOST) and the Single Joint Ethics Review Board (SJERB) which reviews and assesses applications of potential vaccine brands that are eyeing to conduct clinical trials in the country.
After the DOST-VEP and SJERB review the application, it will then be submitted to the Food and Drugs Administration (FDA), which grants the Certificate of Product Registration (CPR) for a vaccine.
Under Executive Order No. 121 signed by President Rodrigo Duterte last December 1, the FDA is also authorized to issue an emergency use authorization (EUA) to Covid-19 vaccines.
With the FDA allowed to issue EUAs, the process of approving COVID-19 vaccines will be shortened from six months to 21 days.
“[Ang FDA,] sinisigurado kung safe ba ang bakuna at kung effective ito. Pinaka importante malaman kung safe ba ang bakuna. Dapat tandaan natin binibigay natin ‘to sa maraming tao na walang sakit so kahit minor side effct kailangan bilangin at alamin natin para alam ng mga tao kung ano ba ang ating inaasahan,” Healthcare Professionals Alliance Against Covid-19 (HPAAC) spokesperson Dr. Antonio Dans said in a press briefing on Monday.
(The FDA ensures that the vaccine is safe and effective. The vaccine must be safe. We should keep in mind that the jabs are given to people who are not sick that’s why we should monitor and consider even the minor side effect so that people will know what to expect.)
“Kailangan din suriin ng FDA kung ano ‘yung major side effects. Para gawin ‘to, inaaral nila ang libo libong pahina ng mga study na sinusumite sa kanila ng manufacturers. Tinitignan din nila ang sample ng mga bakuna,” he added.
(The FDA should also examine the major side effects of the vaccines. To do this, they will probe thousands of pages of studies submitted by vaccine manufacturers. They will also inspect samples of the vaccine.)
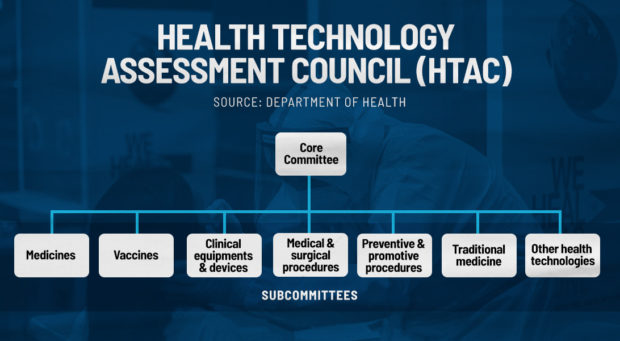
HTAC
Created under the Republic Act 11223 or the Universal Health Care Act, the Health Technology Assessment Council (HTAC) is an independent advisory body that provides “guidance to the Department of Health (DOH) and the Philippine Health Insurance Corporation (PhilHealth) on the coverage of health interventions and technologies to be funded by the government.”
According to Dans, specialists from HTAC would check if the Covid-19 vaccines are worth of its cost. It also analyzes the costs of storage for the vaccines, transport, manpower, and even the energy and water that will be used from procurement to the inoculation process.
“Tinitignan nila at sinisigurado na walang personal, salungat na interest, o conflict na interest na pumapasok sa proseso. Tinitignan nila kung sino ‘yung bumoboto tungkol sa positive recommendation, walang stock holder sa manufacturer, walang merong political interest,” the doctor explained.
(They will make sure that there’s no conflict of interest within the process. They will monitor who will be voting for positive recommendations, there should be no stockholders from the manufacturers or political interest involved in the voting.)
“Sinisigurado nila na ‘pag tinignan nila ‘yung mga bakuna pumapasok lang ‘yung interest ng bayan, hindi interest ng makapangyarihan o may kakayahan,” he added.
(They will guarantee that when a vaccine is chosen, the only interest considered is the public interest, not the interest of those who are wealthy and powerful.)
DOH, NITAG, LGU, NAEFIC
Aside from monitoring the day-to-day caseload of Covid-19 in the country, the DOH along with the group of medical experts from the National Immunization Technical Advisory Group (NITAG), and local government units (LGUs) are tasked to monitor the distribution of the vaccine jabs.
“Once na-approve na ng FDA at na-recommend ng HTAC, sila naman ang tungkulin nila, ano ba ang makatarungan na distribusyon? Sino ba ang uunahin natin?” said Dans.
(Once the FDA and HTAC approved and recommended a vaccine, it is the task of DOH, NITAG, and LGUs to oversee the vaccine distribution. They will decide who should be included in the first batch of vaccination.)
Also included in the core agencies mentioned by the HPAAC is the National Adverse Events Following Immunization Committee (NAEFIC).
Support groups
Dans said that there are also various groups, including expert groups, professional societies, and civil society groups, that are consulted during the process of choosing and approving vaccines.
Also included are the vaccine cluster of the Inter-agency Task Force (IATF) for the Management of Emerging Infectious Diseases, which is headed by the National Task Force (NTF) against Covid-19 chief implementer and vaccine czar Carlito Galvez Jr.
Among the vaccine cluster are:
- Technical Group on Vaccine Evaluation & Selection
- Technical Group on Diplomatic Engagement & Negotiation
- Technical Group on Procurement & Finance
- Technical Group on Vaccine Cold Chain & Logistics Management
- Technical Group on Demand Generation & Communications
- Technical Group on Covid-19 Immunization Program
How do these agencies decide on which vaccine to approve?
Before going through the step-by-step processes of the vaccine approval, it is worth noting that the process is divided into two groups.
The first group consists of the activities under the research and development for the Covid-19 vaccines. Meanwhile, the second group is focused on the tasks of the core agencies in connection with the work results of the first group.
Clinical trials
The vaccine approval starts with the three phases of clinical trials. As explained by Dans, in Phase 1, few volunteers are given jabs of the vaccine to see if there are any significant effects. If Phase 1 is deemed successful, researchers will proceed to Phase 2 where a small clinical trial is conducted. During this stage, some volunteers will be given vaccine shots to see if the vaccine is worth the fund to undergo Phase 3 trials.
Phase 3 clinical trials are most important and the final test before a vaccine is approved. Based on a statement by a team of McGill University medical experts who manage the COVID19 Vaccine Tracker website, in Phase 3 trials “the vaccine is given to tens of thousands of healthy volunteers to determine whether the vaccine protects them from becoming sick.”
“Typically, Phase 3 trials enroll those at highest risk of the disease. If the vaccine proves to be safe and effective throughout these trials, all of the evidence will be reviewed by regulatory agencies who will determine whether to approve it for widespread use,” the statement posted on the website read.
Preliminary data and the EUA
HPAAC spokesperson Dans said that in a usual procedure for vaccine approval, the FDA will have to wait for final data after the three stages of clinical trials before issuing a Certificate of Product Registration (CPR).
However, Dans explained that the country is in an emergency due to many deaths and active Covid-19 cases. “Sa sitwasyon na ito, meron tayong preliminary data. ‘Di pa po tapos yung data, kailangan na nating magdesisyon kung aling bakuna ang gagamitin.”
(In a situation like this, we will have preliminary data. The data is still incomplete but we already need to decide on which vaccine will be used.)
The problem with this is that the FDA will not be able to give CPR or approval to a vaccine if the data is still incomplete.
This is where the EUA enters the process. Yet, Dans clarified that the EUA is not similar to CPR. Under the EUA, only the national government can purchase vaccine doses.
“Ang EUA, ‘di po yan CPR. ‘Di pa rin pwede mag benta ang kumpanya, ‘di pa rin pwede mag kanya -kanyang bili sa emergency use authorization dahil incomplete pa ang data at inaprubahan lang yan ng provisional dahil merong isang national emergency,” the doctor said.
(The EUA is not the same as CPR. Individual or separate purchase [among private sectors and LGUs] are not yet allowed since the data is still incomplete and the process was just approved because there is a national emergency.)
“Ang pwede lang bumili ng gamot [o vaccine] ay ang national government. Kung bibili man ang private sector at ibang mga agency, kailanan dadaan sila sa supply ng national government,” he added.
(Only the national government can purchase the vaccines. If the private sector and other agencies want to procure their own doses, they will have to go through the supply of the national government.)
Final data and Phase 4
Based on Dans’ data, after the added preliminary data and the EUA, there will be a final data which will be reviewed again by the FDA for CPR. Then, Phase 4 or the Post Market Surveillance will be conducted.
During Phase 4, the HTAC will monitor those who were inoculated for any side effects before giving the vaccine a positive recommendation. The recommendation, according to Dans, is like an authorization that is issued for the purchasing and procurement of the vaccines.
Purchase, procurement, vaccination
Once the vaccines are purchased and procured, DOH, NITAG, and different LGUs can distribute and administer vaccines to those included in the priority groups.
Dans added that NAEFIC will monitor the first batch of inoculated Filipinos to include details in the Phase 4 report.
JPV
For more news about the novel coronavirus click here.
What you need to know about Coronavirus.
For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our healthcare frontliners and is still accepting cash donations to be deposited at Banco de Oro (BDO) current account #007960018860 or donate through PayMaya using this link .
Disclaimer: The comments uploaded on this site do not necessarily represent or reflect the views of management and owner of Cebudailynews. We reserve the right to exclude comments that we deem to be inconsistent with our editorial standards.