OPAV’s PBB bares 4 brands used in rapid testing
AP photo
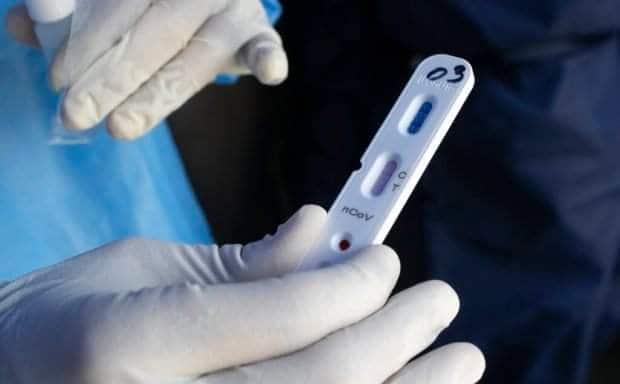
CEBU CITY, Philippines — The Office of the Presidential Assistance for the Visayas’ (OPAV) Project Balik-Buhay (PBB) has revealed the four brands of rapid antibody-based test kits that are used in running the blood samples from the mass testing in the cities of Cebu, Mandaue and Lapu-Lapu.
These test kits brand include the Novel Coronavirus (2019-NCOV) IgM/IgG Antibody Detection Kit of Nanjing Vazyme Medical Technology Co., Ltd; 2019-nCoV Antibody Test of Innovita (Tangshan) Biological Technology Co., Ltd; OnSite COVID-19 IgG/IgM Rapid Test of CTK Biotech; and Biolidics 2019-nCoV IgG/IgM Detection kit of Biolidics Limited.
Nanjing Vazyme Medical Technology C., LTD and Innovita (Tangshan) Biological Technology Co., Ltd are based in China, Biolidics Ltd. is based in Singapore, and CTK Biotech is based in the United States.
Dr. Mary Jean Loreche, chief pathologist of the Department of Health in Central Visayas (DOH-7), said these test kits have the highest specificity and sensitivity yields during their laboratory validation hence, their decision to use these kits in the community testing.
“When I came on board for the technical working group for the community testing, the first thing that I said was: ‘I need a test kit that I have faith [in].’ If you will check in my 30 years of practice, I will not sacrifice results, quality versus something that I do not have faith in,” Loreche said.
Loreche said all four kits are approved by the Food and Drug Administration (FDA).
Read: Economic-health balance sought in deciding Cebu HUCs shift to modified ECQ
Although only the Research Institute for Tropical Medicine (RITM) in Metro Manila is the only center authorized to conduct a validation of the kits, Loreche said they conducted independent validation tests on the kits in order to determine which will be best used for the community testing.
During the validation, Loreche said they tested samples from confirmed coronavirus disease (COVID-19) positive and negative patients in order to determine the specificity and sensitivity rating of the kits. The specificity rating refers to the capability of the kit to detect a “true positive” while the sensitivity refers to the accuracy in detecting “true negative” samples.
Loreche said the sensitivity ratings for these kits are at least 96 percent while its specificity marks are at 99 percent, Loreche said.
The specialist added that with these ratings, the four test kits show a very minimal margin of error in detecting the presence of infection. However, Loreche emphasized that results for the antibody test kits cannot be used as the main diagnostic tool for COVID-19.
Those who will yield a positive result in the antibody-based test will still have to be verified through the polymerase chain reaction (PCR) test, which is considered as the gold standard in diagnosing COVID-19.
As of May 17, the OPAV-initiated PBB has identified 372 persons who participated in the rapid mass community testing that will have to undergo swab testing for PCR test. This is only 1.9 percent of the total samples that have been collected and tested under the PBB. /bmjo
Disclaimer: The comments uploaded on this site do not necessarily represent or reflect the views of management and owner of Cebudailynews. We reserve the right to exclude comments that we deem to be inconsistent with our editorial standards.
