Who is shouldering ‘community’ test costs?
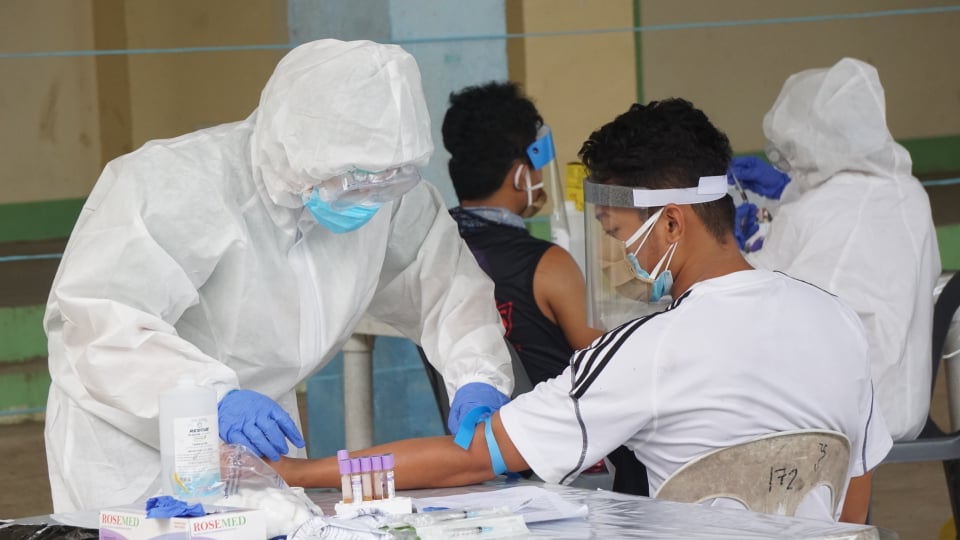
A medical frontliner takes a blood sample during the strategized rapid mass testing in Cebu City. | CDN Digital Photo by Gerard Vincent Francisco
CEBU CITY, Philippines — Costs for procuring the test kits of the ‘strategized community testing’ in Cebu are shouldered by local governments and members of the private sector, local sources confirm on Monday, May 18.
City Legal Officer and Lawyer Rey Gealon recently confirmed that the money needed for the purchase of rapid antibody test kits formed part of the city’s P1 billion war chest against the coronavirus disease 2019 (COVID-19) pandemic.
“Supplemental Budget No. 2, which amounts to P1 billion, serves as our fund for our Response, Relief, and Recovery — our strategies to address the crisis. This includes the purchase of test kits for the strategic mass testing,” said Gealon in Cebuano in a live press conference.
Gealon was referring to the strategized rapid mass testing called the Project Balik Buhay (PBB) initiated by the Office of the Presidential Assistant for the Visayas (OPAV) and the cities of Cebu, Mandaue, and Lapu-Lapu.
READ MORE: ‘Strategic’ mass testing for COVID-19 to be conducted in Cebu, Mandaue
However, it was not mentioned as to how much the city shelled out for the acquisition of at least four rapid antibody test kit brands, all of which were imported.
READ MORE: OPAV’s PBB bares 4 brands used in rapid testing
Aside from Gealon, officials from the Department of Health in Central Visayas (DOH – 7) told the media that ‘arrangements for the laboratories, volunteer workers are with the local government based on agreed guidelines’.
“(What we advised is) that the testing kits should be approved by FDA (Food and Drugs Administration)… We are not the procuring entity, and we never recommended any brand,” DOH – 7 director, Dr. Jaime Bernadas, said.
DOH-7’s chief pathologist, Dr. Mary Jean Loreche, also said in an earlier press conference that OPAV and several members of the private sector were part of PBB.
“Actually (this is) shouldered by the LGUs (local government units) with the participation of OPAV. And also the business sectors are helping out in these,” said Loreche.
PBB, supported by several business groups in Cebu while criticized by some public officials and citizens, was formulated in anticipation for the tri-cities to transition to the ‘new normal’.
READ MORE: Cebu biz groups: PBB is our passport to new normal
Health workers under PBB started collecting blood samples from selected residents in the three cities last May 6, and reported a 75 percent turnout rate on Monday.
On May 14, Presidential Spokesperson Harry Roque announced that LGUs would be allowed to use their funds for the procurement of rapid test kits.
READ MORE: LGUs now allowed to purchase COVID-19 rapid test kits
This despite the DOH and FDA’s earlier rejection to endorse rapid anti-body test kits due to their findings that these could give inaccurate results, and a department memorandum circular No. 2020- 061 dated March 31 stating that public funds should not be used in the purchase of rapid COVID-19 test kits.
Then Malacañang on April 15 greenlighted and decided to purchase rapid antibody test kits to give the country’s much needed massive screening of COVID-19 a boost./dbs
Disclaimer: The comments uploaded on this site do not necessarily represent or reflect the views of management and owner of Cebudailynews. We reserve the right to exclude comments that we deem to be inconsistent with our editorial standards.
