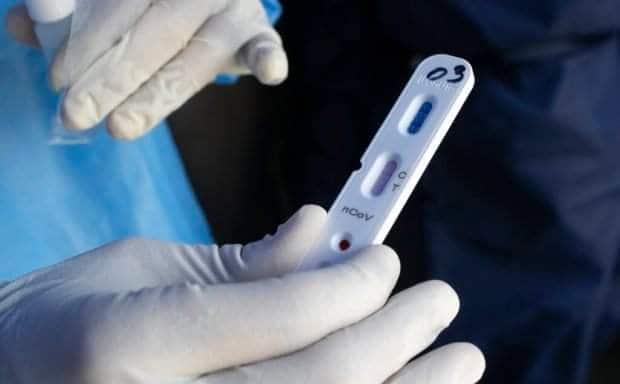
Rapid antibody test kits: A closer look
AP photo
CEBU CITY, Philippines — Around the globe, coronavirus disease 2019 (COVID-19) test kits are flying off from every biotechnological and medical technology firm as countries scramble to screen everyone for possible infection.
There are two types of widely known and used COVID-19 test kits — these are the real-time reverse-transcript polymerase chain reaction (RT-PCR), and the rapid antibody diagnostic tests (RDT).
In Cebu, interest and scrutiny were cast upon the latter especially when the ‘strategized community testing’ for the cities of Cebu, Mandaue, and Lapu-Lapu began on May 6.
The mystery surrounding the brands of RDTs being used for Project Balik Buhay (PBB), the name of the strategized testing, has finally cleared after they were revealed to the public on Monday, May 18.
READ MORE: OPAV’s PBB bares 4 brands used in rapid testing
Initially rejected by the Department of Health (DOH) and the Food and Drug Administration (FDA) for producing inaccurate results, however, RDTs are now green-lighted to serve as ‘conduits’ to the gold standard RT-PCR.
On May 14, the Malacañang even announced that local governments can use their funds for the acquisition of these particular diagnostic tools.
READ MORE: LGUs now allowed to purchase COVID-19 rapid test kits
But in compliance with DOH’s guidelines, swab samples should be extracted from individuals, who tested positive of antibodies before they can be officially included in the country’s tally of COVID-19 cases.
RDT vs RT-PCR
RT-PCR is considered as ’the gold standard’ in detecting COVID-19 infection as it determines through oral and nasal swab samples whether a person has contracted SARS-CoV-2, the virus that causes COVID-19.
RDTs, on the other hand, usually employ the ‘colloidal gold method’ that help health experts identify if blood samples extracted for examination are present with antibodies.
According to DOH in Central Visayas, antibodies either appear during infection (IgM) or upon recovery (IgG).
PBB and RDTs
PBB is funded with money from the local governments of the three cities, members of the private sector, and the Office of the Presidential Assistant for the Visayas (OPAV), which spearheaded the program.
READ MORE: Who is shouldering ‘community’ test costs?
In the tri-cities, it was revealed that at least four brands of RDTs had been utilized to collect the ‘much-needed data’ for local officials to create the COVID-19 prevalence map.
The map, in turn, will serve as their basis to implement policies and regulations for the ‘new normal’.
All four brands of RDTs were among the 47 products approved by FDA for non-commercial use which means selling these tools are prohibited, and all are imported.
CDN Digital compiled some information pertaining to the manufacturers, obtained from their official websites and company profiles.
1. Product Name: 2019-nCoV ANTIBODY TEST (COLLOIDAL GOLD)
Manufacturer and Address: Innovita (Tangshan) Biological Technology Co., Ltd. –No. 699 Juxin Street, High-Tech Industrial Development Zone, Qian’an 064400, Hebei, China.
– It is one of the first RDT kits approved by FDA. Founded in 2006 and first headquartered in Beijing, Innovita Biological Technology Co. specializes in research and development (R&D), particularly in-vitro diagnostic (IVD) tools.
The United States FDA defines IVD tools as instruments to aid tests conducted on samples such as blood or tissues extracted from a human body.
2. Product Name: OnSite COVID-19 IgG/IgM Rapid Test
Manufacturer and Address: CTK Biotech, Inc. –13855 Stowe Dr. Poway California, USA
– The company is based in San Diego, California, and manufactures diagnostic tools. There are only six US-based manufacturers of RDTs approved by the FDA in the Philippines.
3. Product Name: BIOLIDICS 2019-nCoV IgG/IgM DETECTION KIT
Manufacturer and Address: Biolidics Limited. – 37 Jalan Pemimpin, #02-07, Mapex, Singapore
– Biolidics was incorporated in 2009, and is now a listed firm on the Singapore Stock Exchange involved in ‘medical technology and diagnostic solutions’.
4. Product Name: NOVEL CORONAVIRUS (2019- NCOV) IgM/IgG ANTIBODY DETECTION KIT (COLLOIDAL GOLD METHOD)
Manufacturer and Address: Nanjing Vazyme Medical Technology Co., Ltd – Level 1-3, Bldg. C2, Red Maple Sci-Tech Park, Kechuang Road, Nanjing China
– Vazyme Biotechnology is based in Nanjing, China, and focuses on ‘clinical diagnosis, molecular diagnostics, high-throughput sequencing, and life science research’./dbs
Disclaimer: The comments uploaded on this site do not necessarily represent or reflect the views of management and owner of Cebudailynews. We reserve the right to exclude comments that we deem to be inconsistent with our editorial standards.
