COVID – 19 in Cebu City: ECQ and mass testing
Part 2 of a Special Report on Cebu City's COVID-19 crisis
AP photo
Part 2 (Conclusion)
It was still two days since Cebu City was placed under Enhanced Community Quarantine (ECQ) and public officials then were racing to address the public’s clamor for a more efficient way of distributing quarantine passes.
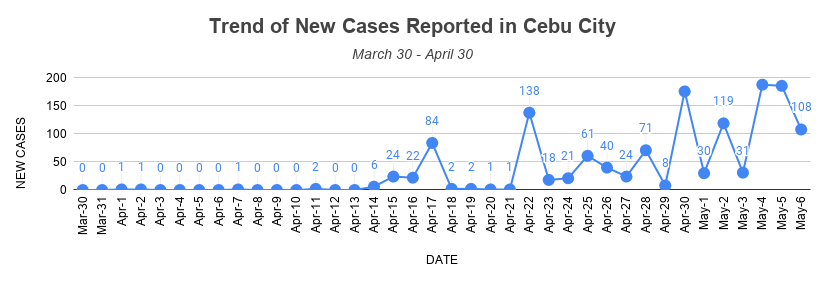
On March 30, the city government led by Mayor Edgardo Labella conducted their regular press conference to provide updates on the coronavirus disease 2019 (COVID-19) situation in the city. It was the city’s second day under ECQ, and the cases at that time were only 20.
On that very same media briefing, aired through live television, radio and even on social media, the city’s health officer, Dr. Daisy Villa, sounded the alarm that the virus could potentially spread to urban poor communities.
“Most of the patients, who tested positive of COVID-19, belong to Class A and B. What we worry (about) are those under Classes C and D, and (those) living in the slum areas. Once this virus spreads there, it would be hard to contain,” were Villa’s words.
The City Health Department (CHD)’s contact tracing for the city’s first 20 COVID-19 patients was underway by then.
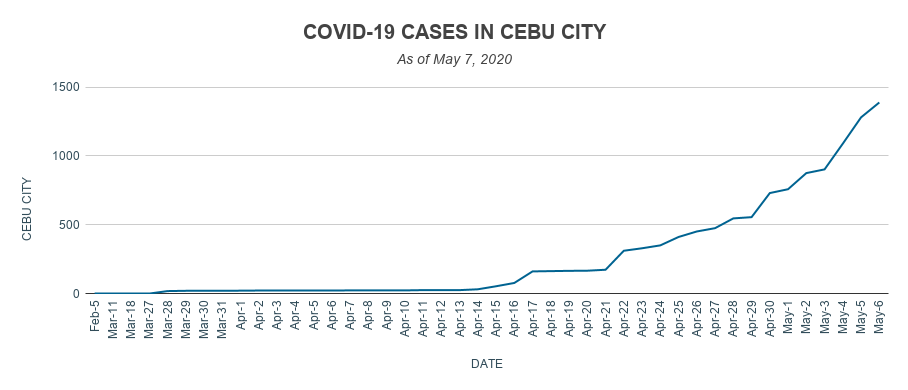
From 20 on March 30, the number of total confirmed COVID-19 cases in Cebu City has skyrocketed to 1,388 on May 6.
This May, the Cebu City government will be participating in a ‘strategic’ mass rapid testing together with the cities of Mandaue and Lapu-Lapu under the ‘Project Balik Buhay’ program initiated by the Office of the Presidential Assistant for the Visayas (OPAV)
READ: ‘Strategic’ mass testing to be conducted in Cebu, Mandaue and Lapu-Lapu
More than 23,000 individuals in Cebu City alone have been identified to undergo the test, which is a deviation from the standard real-time polymerase chain reaction (RT-PCR) techniques.
The ‘Project Balik Buhay’ program was formulated in the hopes that Metro Cebu’s three largest cities will relax their enhanced community quarantine (ECQ) protocols, and transition to a general community quarantine (GCQ) or also known as the ‘new normal’ by May 21.
The rapid test kits were tagged as conduits to the already existing RT-PCR tests being conducted in Cebu City.
Health experts from the Department of Health in Central Visayas (DOH – 7) dubbed the initiative as a new strategy to determine the level of infection in the island’s communities, which in turn could provide the necessary insights and data that policymakers needed to evaluate the prevailing ECQ.
Cebu City was the first local government unit in the province to implement ECQ. On March 28, the city’s ECQ effectively paralyzed mass transportation and led to the suspension of several ‘non-essential’ business establishments.
RT-PCR vs. rapid antibody tests
RT-PCR tests require swab samples taken from suspected COVID-19 patients both through nasopharyngeal (nasal) and oropharyngeal (throat). These specimens will then be examined with the use of a PCR machine.
For Cebu, the subnational laboratory (SNL) in Vicente Sotto Memorial Medical Center (VSMMC) can accommodate a daily average of 500 tests. It has started running COVID-19 tests since March 19.
The mass testing conducted in Cebu City since April 14 and April 15 utilizes RT-PCR laboratory methods. Data from the City Health Department (CHD) showed that a total of 6,389 samples as of April 30 have been tested using this technique.
The DOH has made it a rule that only specimens examined through RT-PCR techniques in certified molecular laboratories in the country, such as the VSMMC, can be part of the nationwide tally of COVID-19 patients.
Malacañang on April 15 purchased rapid test kits to augment the Philippines’ much-needed boost to expand its COVID-19 test capabilities. This despite earlier warnings from DOH’s central office, and the Food and Drugs Administration (FDA) that rapid test kits could produce inaccurate results, possibly leading to false negatives.
As a precaution, the DOH advised local officials and medical facilities nationwide carrying COVID-19 tests that results yielded by rapid antibody tests should still undergo further verification through RT-PCR techniques.
READ: Rapid test kits get Duterte nod despite DOH rejection
The Inter-Agency Task Force on Emerging Infectious Disease (IATF-EID), the national government’s decision-making body on the COVID-19 crisis, has ordered that scientific evidence should serve as the basis for downgrading ECQ.
READ MORE: IATF: ECQ to stay in Cebu, 8 other areas until May 15
As a result, local officials here in Cebu came up with the Project Balik Buhay program which consists of a step-by-step method in determining if an area can transition to the ‘new normal’.
The program will take 10 percent of a barangay’s population as a sample, and these individuals will have to undergo rapid COVID-19 tests. And those tested will remain in their houses, regardless if the results of their tests showed whether or not they have been infected with COVID-19.
But for individuals who will yield positive results during the tests, they will undergo the ‘gold standard’ RT-PCR method of COVID-19 test for further verification. They will also be placed in isolation centers, where existing asymptomatic coronavirus patients are staying, as they wait for their official results.
Collated data from the rapid tests conducted will then be analyzed for local officials to plot which areas in their territories can ‘safely’ transition to the new normal. This is done through a zone-tagging classification system.
In Cebu City, the strategic mass rapid test was pushed back from May 4 to May 6 as local officials continue to iron out procedures of carrying out such program.
The DOH – 7 assured the public that the rapid tests to be used for the Project Balik Buhay program are those approved by the FDA. The country’s regulatory board of consumer products gave clearance to a total of 16 kinds of COVID-19 test kits.
READ: Experts support use of rapid antibody-based testing
Word of Caution
But before this development, experts from the University of the Philippines (UP) who are behind some of the country’s widely accepted policy papers on responding to the COVID-19 crisis, cautioned local officials and national government agencies on the lifting of the ECQ.
READ: ‘Pandemic is far from over in Cebu’ – experts
In a report published on April 22, researchers warned the public not to take the pandemic lightly as they have observed a shift in its dynamics – particularly on the age distribution among those who were infected.
They added that the COVID-19 situation in Cebu ‘is far from over’, and reiterated these findings and the calls for other areas in the Philippines to conduct mass testing in a more recent study made on April 30.
Scientists stated that the ECQ in Cebu must stay in place and be strictly implemented “until the spread of the pandemic has been managed.”
“The province was enjoying a flat curve until April 15 when new cases of COVID-19 started to appear. This highlights again the importance of mass testing and available health facilities. If strict protocols are not put in place, any region or province is susceptible to an outbreak at any given time, as long as the SARS-Cov2 virus is still lingering,” the study said. /dbd
Disclaimer: The comments uploaded on this site do not necessarily represent or reflect the views of management and owner of Cebudailynews. We reserve the right to exclude comments that we deem to be inconsistent with our editorial standards.